


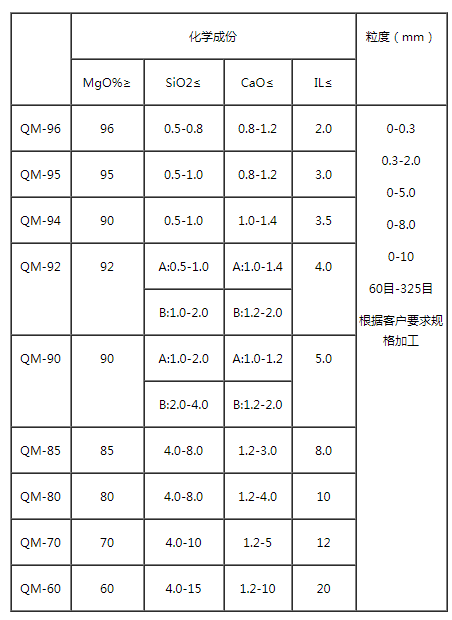
Caustic calcined magnesia (Caustic calcined magnesia) 12 Calcined magnesite, brucite and magnesium hydroxide extracted from seawater or brine at about 800~1000℃ to decompose CO2 or H2O to obtain light calcined magnesia powder. According to chemical composition, light burned magnesia powder is divided into nine grades: QM-96, QM-95, QM-94, QM-92, QM-90, QM-87, QM-85, QM-80, QM-75. Light burnt magnesia powder
The light burnt magnesia powder is light yellow and light brown powder, the particle size is mostly below -100 mesh, the periclase crystal is very small (<3μm), the true specific gravity is 3.07~3.22, the bulk density is 0.8~1.2g/cm3, and the refractive index is 1.68 ~1.70, large lattice constant (α=0.4212), many defects, crisp texture, pore structure, high reactivity, easy to undergo solid-phase reaction or sintering, and harden by reacting with water to produce Mg(OH)2. Bonding ability. The light-burned magnesia powder calcined from magnesite has a volume shrinkage of about 5%; usually 3% to 5% CO2 remains due to incomplete decomposition.
Light burned magnesia is mainly used to manufacture cementitious materials, such as magnesium-containing cement. Thermal insulation and sound insulation building materials, and can also be used as ceramic raw materials. After chemical treatment of light burned magnesia, it can be made into a variety of magnesium salts and used as medicine. Magnesium ball rubber, man-made fiber, papermaking and other raw materials. Dead burned magnesia. Most of the refractory materials used in metallurgy are used to make magnesia bricks. Chrome magnesia bricks. Magnesium sand. Metallurgical powder. Fused magnesium oxide is mainly used as Smelting special alloy steel. Non-ferrous metals and precious metals in the middle and high frequency induction furnace lining. Magnesium crucible. It can also be used as high temperature electrical insulation material. The source of light burned powder is calcined magnesite.